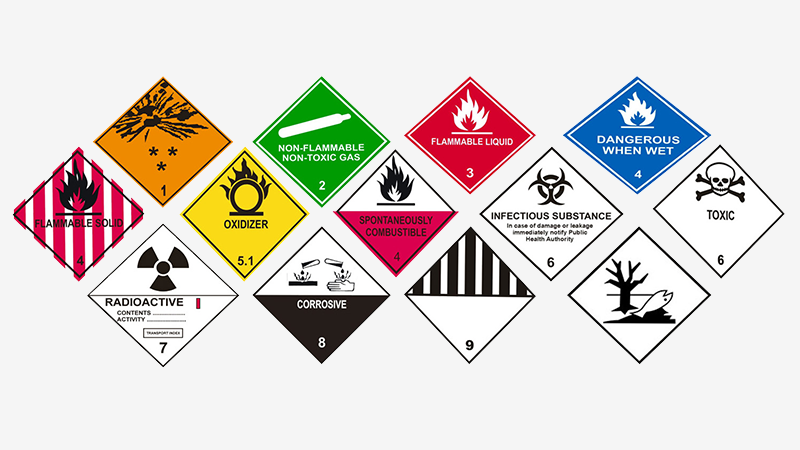
Our services

Why do clients choose us?

Other services

EU Ecolabel
Documentation at any stage of the application, recipe control, tests.
Nordic Swan
Documentation at any stage of the application, recipe control, tests.
Eco Right Way
Documentation at any stage of the application, recipe control, tests.
Contact us